| No. |
DIAGRAM |
DESCRIPTION |
| A1 |
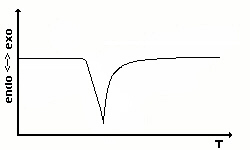 |
The most simple differential temperature curve of an
endothermal process
is a first order phase transition. Each substance showing such a
behavior
can be used as a calibration standard. |
| A2 |
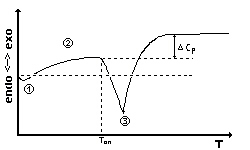 |
A theoretical melting curve: It begins with a starting turn
due to
the amount of reference substance(1). Then, the curve shows the course
of heat capacity of the sample(2). While the substance is melting the
slope
of the signal depends on the heating rate. At point (3) the substance
is
completely molten and exponential relaxation appears. If the heat
capacity
Cp of the sample has changed during the phase transition the
base line shifts to a different height. The melting temperature is
observed
as Tonset. |
| A3 |
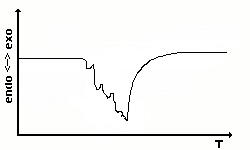 |
The sample is coarse-grained. To obtain a sharp signal the
substance
has to be pulverized. Sometimes, similar effects can be found which are
caused by bubble formation in molten probes. |
| A4 |
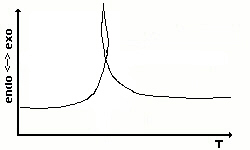 |
An enveloped curve can occur when a vehement chemical
reaction takes
place. In this case, we have to chose the timescale as x-axis and
indicate
the temperature as a further line in the diagram. |
| A5 |
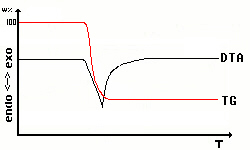 |
Watching chemical reactions involving the atmosphere like
decomposition
or synthesis it is always interesting to use differential thermal
analysis
and thermal gravimetrics simultaneously. This is a famous way to get
ideas
about chemical reactions and to distinguish them from phase
transitions.
Note that the y-axis has two different scales at a time - differential
temperature and weight percent. |
|
And now: |
Some original diagrams |
| 1 |
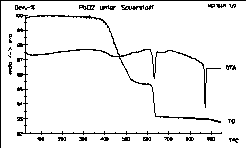 |
Thermal decomposition of PbO2 under pure oxygen
atmosphere.
Sample of 53 mg with 4 K/min. Red lead forms at 400°C and
decomposes
at more than 620°C under this conditions! Look at the sharp melting
peak of PbO.
Step 1: 3 PbO2 -> Pb3O4 + O2
Step 2: Pb3O4 -> 3 PbO + 1/2 O2 |
| 2 |
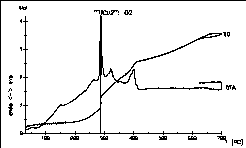 |
The oxidation processes of an intermetallic alloy "TlCu2"
under
oxygen was investigated to find the optimum conditions for synthesizing
TlCu(CuO2). After evaluation of the data we where able to
collect
lots of crystals! This model substance was discovered by Arnold
Adam, Claudia Felser-Wenz, H.-U. Schuster and R. Hoppe in 1991.
Ref.:
Z.
Anorg. allg. Chem. 605 (1991) 157-62. |
| 3 |
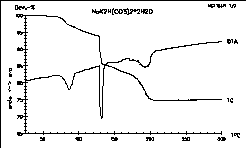 |
The thermal behavior of NaK2H(CO3)2
*
2 H2O under argon, a beautiful example for a three step
decomposition.
This Hydrogencarbonate has been prepared in the Adam's
Group at the University of Cologne by Vytas Cirpus.
Ref.: A. Adam, V. Cirpus
"Die ersten gemischten Alkalimetallhydrogencarbonate NaA2[H(CO3)2]
*
2 H2O mit A = K, Rb" 25. GDCh-Hauptversammlung,
Münster,
Coll. Abstr. (1995) 483. |
| 4 |
 |
NaK5[H(CO3)2]2
measured
under argon: at first sight it seems to be a one step decomposition,
but
actually two steps overlap. Pay attention to the change of slope in the
TG line! Vytas did it again.
Ref.: V. Cirpus, A. Adam
"NaK5[H(CO3)2] - das erste wasserfreie
Hydrogenbicarbonat" Z. Kristallogr., Suppl. Issue 15 (1998) 28. |
| 5 |
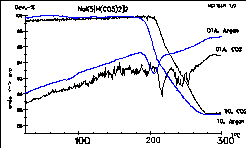 |
The same substance as above. The black lined measurement was
taken
under CO2 atmosphere. You will often observe troubled
dT signals with carbon dioxide because of interactions between gas and
substance surface. |
| 6 |
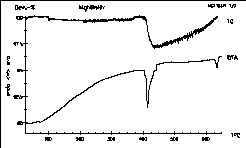 |
MgNiInHx - how much hydrogen could Axel Karge
from H.-U.
Schuster's stuff store in his compound? Nearly three weight percent!
The
argon atmosphere was not totally free from oxygen as the TG line shows.
Axel Karge, Dissertation Köln 1997. |
| 7 |
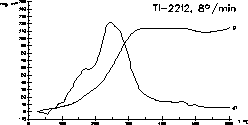 |
The synthesis of the high TC superconductor
Tl-2212 succeeds
by slow oxidation of a chilled alloy of the metallic components. The
oxidation
of a well prepared alloy proceeds in one step under control of the
thermal
parameters.
H.-U. Schuster, J. Wittrock: J. Thermal. Anal. 39 (1993) 1397-1401. |
| 8 |
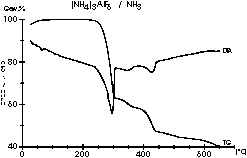 |
DTA/TG measurement under ammonia! Meike Roos investigated the
courses
of the ammonolysis reactions of the ammonium hexafluorometalates (NH4)3MF6
(M = Al, Ga, In) with the aid of in-situ powder diffractometry and
differential
thermal analysis. After finding the optimum measuring parameters we got
these amazing diagrams which respond great to the results of the other
analytical investigations. This is the Al compound. |
| 9 |
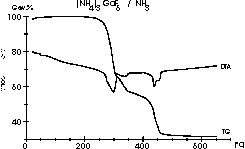 |
A complex scheme for the reactions between (NH4)3GaF6
and NH3 forming many intermediates and resulting in GaN is
described
in:
Meike Roos, Jörg Wittrock, Gerd
Meyer, Silvia Fritz and Joachim Strähle:
Z. Anorg. Allg. Chem. 626 (2000) 1179-1185. (Diagram 8, 9
and
10)
Under 1 atm ammonia it looks quite simple so...
|
| 10 |
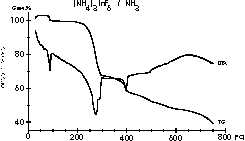 |
A bucket of questions and results - the courses of the
ammonolysis
of (NH4)3InF6 are complex and full of
surprises as Meike Roos and Gerd Meyer showed in Z. Anorg. Allg.
Chem.
625 (1999) 1839. |
| 11 |
 |
Low temperature thermal analysis near the melting point of
ice is quite
an expenditure. Our new device - available now at the FTA
- makes it easy to draw phase diagrams. A prototype has been developed
in the Adam's Group
at
the University of Cologne.
The system K2CO3 / H2O in the near
of the cryohydratic point is shown. Consider that the curves shown are
drawn as been measured - no flattening or adjusting of the base line
has
been necessary! |
| 12 |
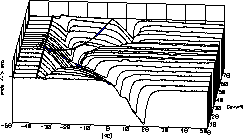 |
Our title picture shows the DTA curves of the system K2CO3
/ H2O in the range from 0 to 75 weight % potassium
carbonate.
The two component regions appear as highland areas between the valleys
of phase transition.
For reference see our Phase Analysis Page. |
| 13 |
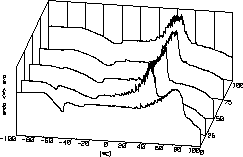 |
Arnold Adam
and his
group investigate the chemistry of carbonates and hydrogen carbonates
of
alkali and alkaline earth metals and in newer times peroxide containing
compounds as well. The line in front shows melting and decomposition of
Perhydrole while the lines behind contain amounts of KHCO3
resulting
in lower decomposition temperatures. The dizziness of the lines results
from bubble formation. Each bubble gives a little peak. |
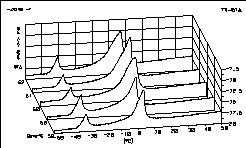
| 14 |
 |
Wegscheiderit Na2CO3 * 3 NaHCO3
-
was the theme of this measurement. The Na2CO3
/ NaHCO3 / H2O system is complex and includes
Trona
Na2CO3 * NaHCO3 * 2 H2O
as
well. See the displacements of the base lines resulting from the
different
heat capacities of the samples. |
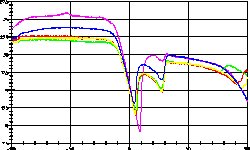
| 15 |
 |
Upside down - a part of the K2CO3 / H2O
diagram
is shown here. Sometimes the beginnings of the peaks appear more
clearly when you choose this look. |
| 16 |
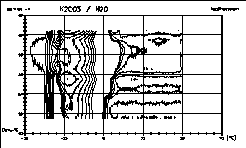 |
Iso(differential)thermal lines of the diagram No. 12 give an
interesting
view of the well known phase diagram. On single curves the anomalies
observed
could be interpreted as artifacts. But if nearly a hundred curves
result
in a coherence it is worth to take a closer look. We will report soon. |
|
to be continued... |
Thanks to all colleagues for the good time in Cologne! |


















